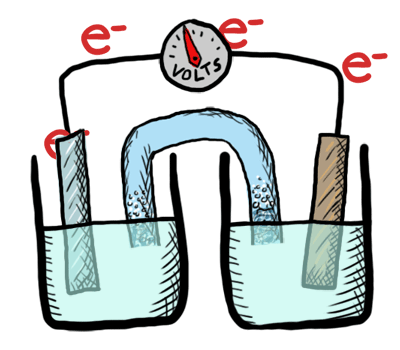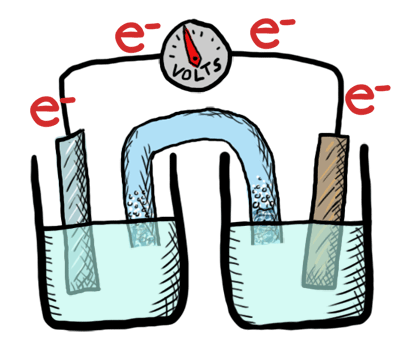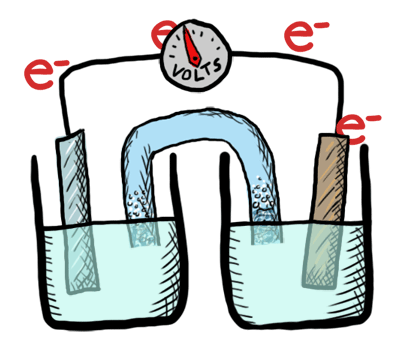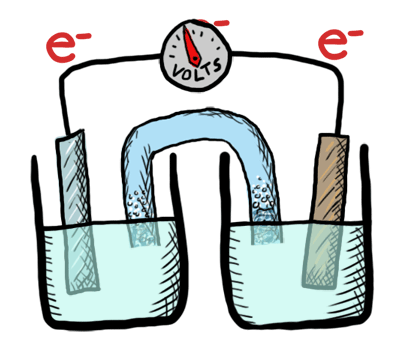
An electrochemical cell converts chemical potential energy into electrical energy. This is accomplished by two electrodes of different chemical potential placed in an electrolyte, resulting in a potential difference between the two electrodes and generation of current electricity. Once all the chemicals in the cell are exhausted—converted to products—the cell no longer generates electrical current. At this point, the cell must either be replaced or recharged. An exhaustible cell is called a primary cell, and a rechargeable cell is called a secondary cell. Several cells connected in series are called a battery. The batteries in your car, laptop and smart phone are all composed of secondary cells. The batteries we use for flashlights and toys—provided they aren’t rechargeable—are composed of primary cells. Note that secondary cells can only be recharged a finite number of times.
This lab was conducted as a demonstration followed by a lab where students created their own electrochemical cell and tested the voltage. Students labeled the schematic diagram of an electrochemical cell and recorded notes as well.








